Automated COVID-19 Workflows.
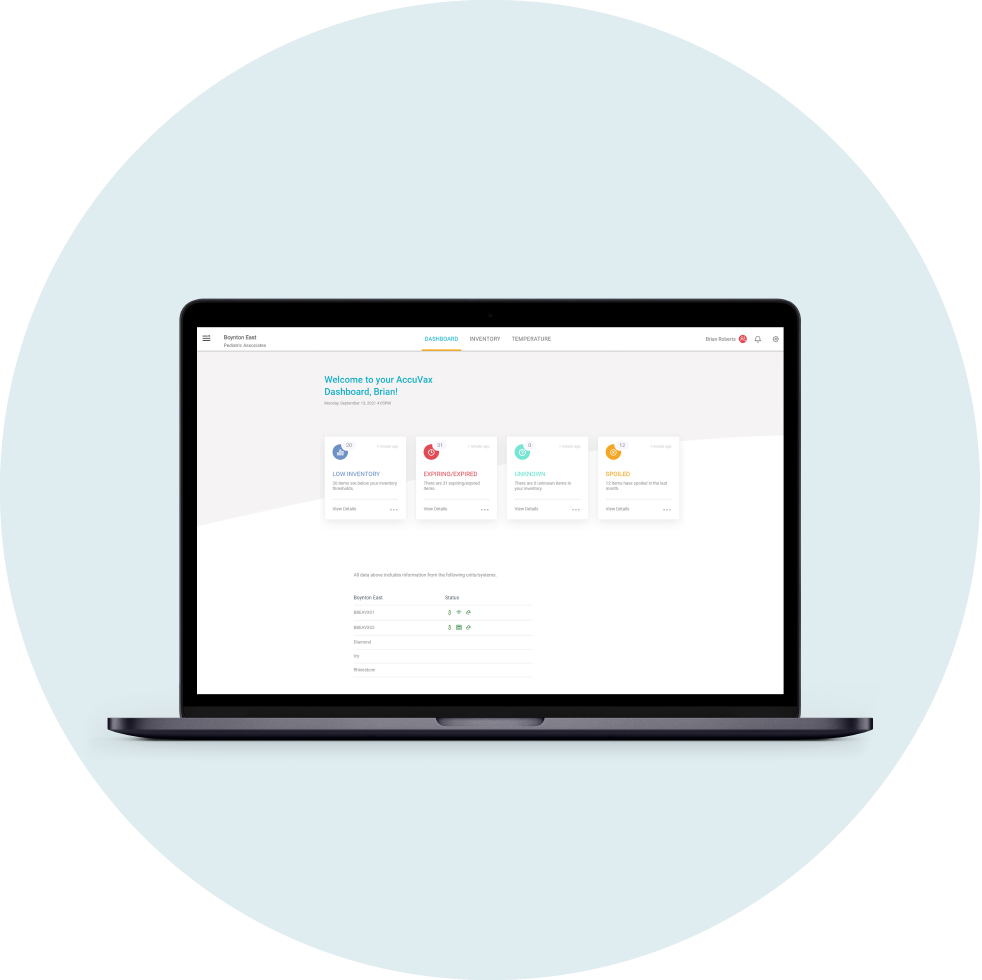
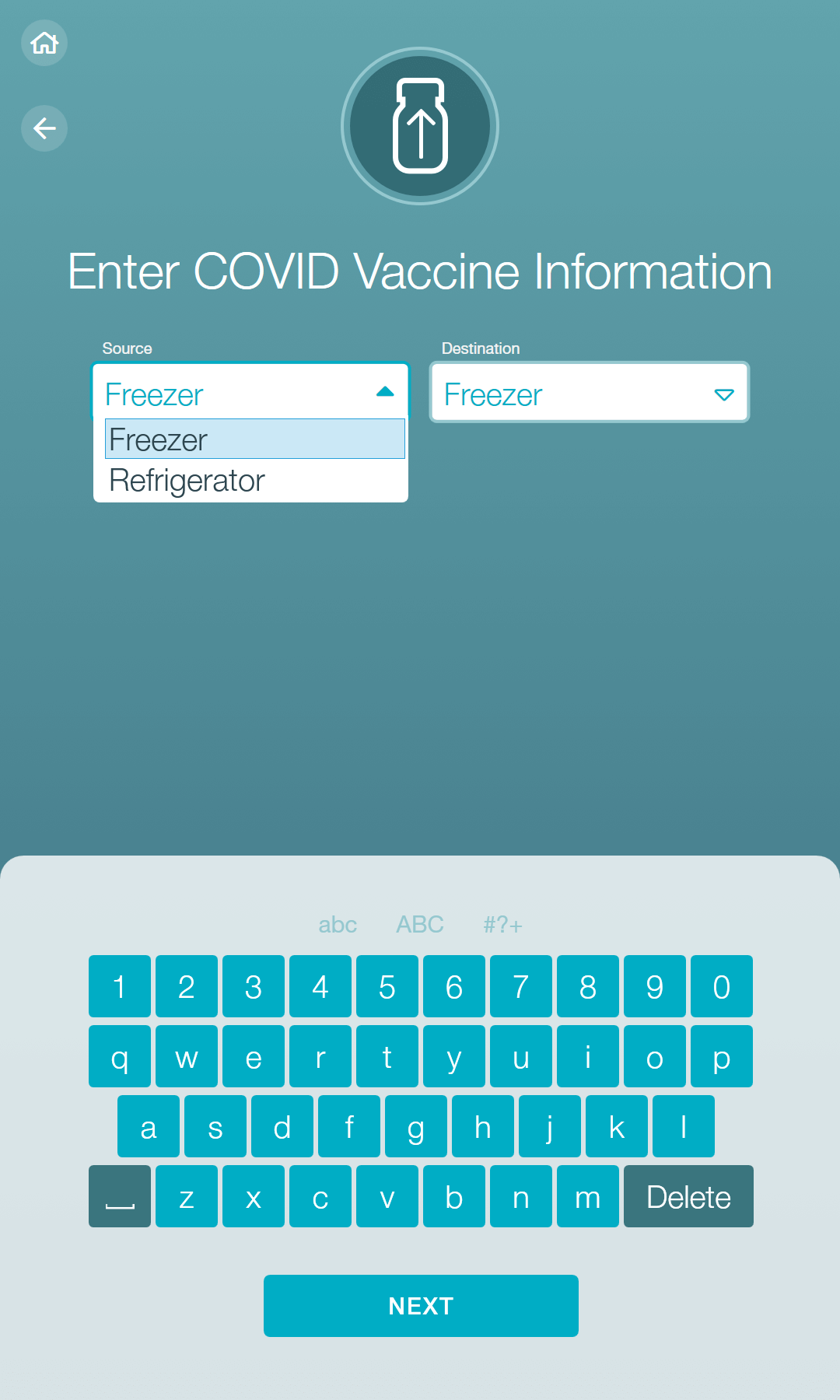
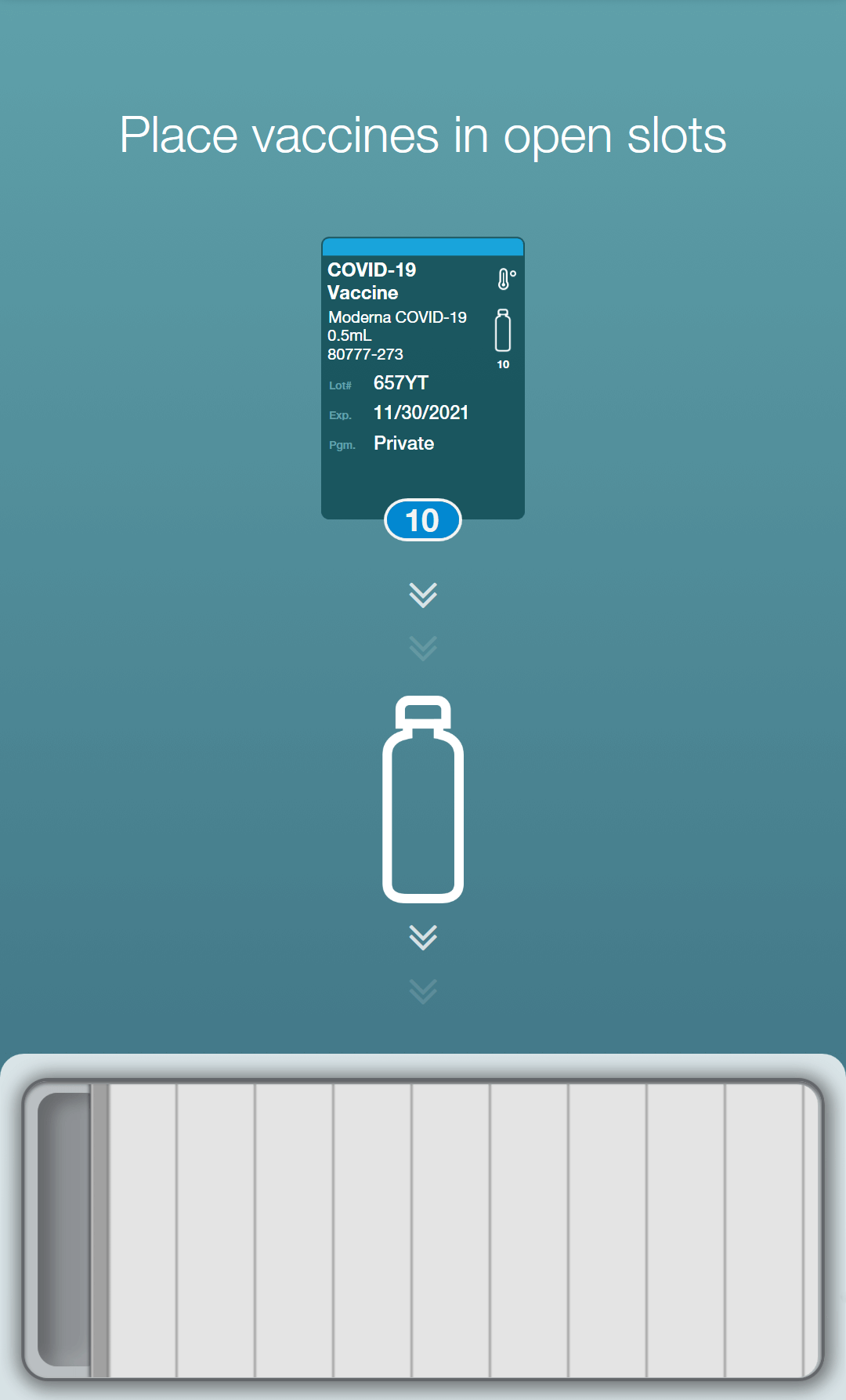
The AccuVax Vaccine Management System is purpose-built for vaccine storage and handling. This technology guarantees temperature control for frozen and refrigerated vaccines within the same unit and includes a built-in battery pack to maintain temperatures for 8 hours after a power outage. In addition, built-in workflows help you manage the complexities of all three COVID-19 vaccine brands and their varying storage requirements and expiration needs. These COVID-19 workflows simplify the complexities by automatically tracking puncture times, beyond use dates, and expiry across multiple temperature storage conditions.
AccuShelf Temperature Monitoring System
Maintain Cold Chain Storage Protocols.
The AccuShelf Inventory Management System can also simplify COVID-19 workflows and monitor multiple cold storage units including ultralow freezers, freezers, and refrigerators. A wireless scanner allows you to record the lot, expiry, and dose of every vaccine, while the integrated digital temperature loggers provide real-time temperature monitoring. With the same COVID-19 workflows, you can identify each temperature location of every COVID-19 vaccine dose and automatically track puncture times, beyond use dates, and expiry.

CDC & State COVID-19 Vaccine References
The CDC has released the COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations along with additional guidelines for vaccination plans by state:
- Illinoispdf icon
- Indianapdf icon
- Iowapdf icon
- Kansaspdf icon
- Kentuckypdf icon
- Louisianapdf icon
- Mainepdf icon
- Marshall Islandspdf icon
- Marylandpdf icon
- Massachusettspdf icon
- Michiganpdf icon
- Micronesiapdf icon
- Minnesotapdf icon
- Mississippipdf icon
- Missouripdf icon
- Montanapdf icon
- N. Mariana Islandspdf icon
Whitepaper: Effective Vaccine Management for Public Health. Improving immunization efforts with the AccuVax Vaccine Management System
Learn solutions to address the many challenges faced by healthcare providers surrounding vaccine management, protecting vaccine potency, improving efficiencies, reducing costs, and increasing immunization efforts.