The COVID-19 vaccinations are no different from any other vaccine in that they have specific safety standards for safe administration and efficacy. All staff members at your practice who will be administering these shots should receive proper training on immunization guidelines. Training should cover everything to know about how to administer COVID-19 vaccine such as how to prepare it properly prior to injection. What actions need take place if there’s an adverse reaction during inoculation? It’s imperative to patient safety and waste-management that all guidelines are followed.
These COVID-19 vaccine administration guidelines are intended to provide a foundational understanding of the requirements for the approved COVID-19 vaccines. When in doubt, contact the manufacturer or your local or state health department.
Administering the COVID-19 Vaccine
After safely receiving and storing COVID-19 vaccines at your practice, you can start to administer vaccines to your patients according to the phased allocation plan. Following vaccine administration guidelines is essential to preventing vaccine waste from incorrect preparation and to ensuring a safe and effective immunization experience for patients.
While strict aseptic practices are key to any vaccine administration process, other best practices will vary depending on the COVID-19 manufacturer. The three vaccinations that are currently authorized (Pfizer-BioNTech, Moderna, and Johnson & Johnson) are not interchangeable, which means it’s critical to follow the vaccine administration guidelines for the vaccines you are using.
Preparing Vaccines
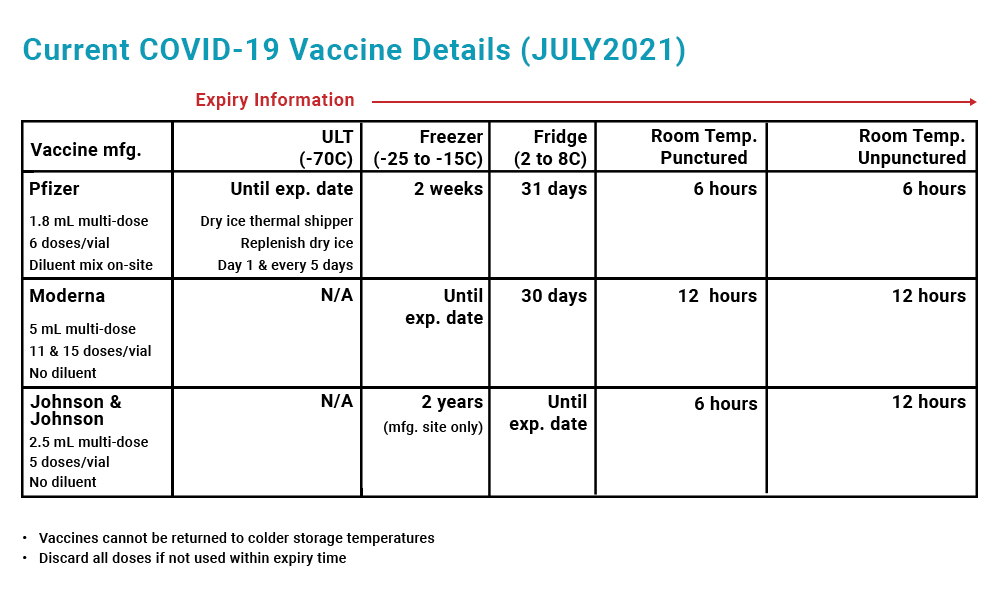
The storage temperatures for COVID-19 vaccines vary from refrigerated (between 2 and 8°C) through frozen (between -15 and -25°C) to ultra-cold (between -60 and -80°C). The vaccines also vary in whether they require diluents.
Pfizer-BioNTech
Pfizer-BioNTech vials need to be thawed to room temperature before preparation. This can be achieved by:
- Transferring vials to a refrigerator at 2 to 8°C for 3 hours where they can be used for up to 31 days; OR
- Leaving vials at room temperature (up to 25°C) for 30 minutes if they need to be administered immediately
Vaccines must be reconstituted within 2 hours and cannot be transferred back to the ultra-cold storage unit.
To reconstitute the vaccine, use only the diluent specified by the manufacturer and included in the mixing kit. Using the wrong diluent will render the vaccine ineffective. The diluent should be stored at room temperature. After adding the diluent, do not shake the vial. The vaccine should become an off-white suspension.
Moderna
Moderna vials must also be allowed to thaw before use, either by:
- Transferring vials to a refrigerator at 2 to 8°C for 2 hours and 30 minutes where they can stay for up to 30 days; OR
- Leaving vials at room temperature, between 15 to 25°C, for 1 hour
Vaccines cannot be refrozen after they have been thawed. Once the vials have thawed, swirl them gently and between each withdrawal. The vaccine should become an off-white suspension. Do not shake or dilute the Moderna vaccine.
Johnson & Johnson
Johnson & Johnson vials are ready to be prepared after being removed from the refrigerator. Holding the vials upright, swirl them gently for 10 seconds and between each withdrawal. The vaccine should appear colorless to slightly yellow, and become a clear to very opalescent suspension. Do not shake or dilute the Johnson & Johnson vaccine. If any vials are shaken, contact the manufacturer.
Special Considerations
Prior to administering a COVID-19 vaccine, be sure to check whether the patient has any allergies and cross-reference these allergies with the vaccine ingredients list. Do not administer the Pfizer-BioNTech, Moderna, or Johnson & Johnson vaccines to patients with a known history of severe allergic reactions.
Patients with underlying medical conditions who do not have contraindications to vaccines may receive a COVID-19 vaccine. Similarly, pregnant and breastfeeding women are advised to receive a COVID-19 vaccine when they are eligible.
The patient’s age may determine their eligibility for a COVID-19 vaccine:
- The Pfizer-BioNTech vaccine can be administered to patients aged 12 and over
- The Moderna vaccine can be administered to patients aged 18 and over
- The Johnson & Johnson vaccine can be administered to patients aged 18 and over

Safe Vaccine Administration Guidelines
Use an alcohol-based waterless antiseptic hand rub or soap and water to sanitize your hands before administering vaccines and between each patient consultation.
The Centers for Disease Control and Prevention (CDC) also recommends wearing personal protective equipment (PPE) when administering a COVID-19 vaccine. Wear a medical face covering and not a cloth mask. You may also use eye protection and gloves.
Pfizer-BioNTech
Before administering the Pfizer-BioNTech vaccine, ensure there is a dosing volume of 0.3 milliliters in the vial. Do not use the remaining vaccine in a vial if it contains less than the full dose. Check that the vaccine in the syringe has no particulates and or discoloration. If particulates or discoloration are present, do not use the vaccine. Dispose of it safely.
If the vaccine passes the visual inspection and contains the correct dosage, administer intramuscularly.
Moderna
Before administering the Moderna vaccine, ensure there is a dosing volume of 0.5 milliliters in the vial. Do not use the remaining vaccine in a vial if it contains less than the full dose. Check that the vaccine in the syringe has no particulates and is not discolored. If particulates or discoloration is present, do not use the vaccine. Dispose of it safely.
If the vaccine passes the visual inspection and contains the correct dosage, administer intramuscularly.
Johnson & Johnson
Before administering the Johnson & Johnson vaccine, ensure there is a dosing volume of 0.5 milliliters in the vial. Do not use the remaining vaccine in a vial if it contains less than the full dose. Check that the vaccine in the syringe has no particulates and is not discolored. If particulates or discoloration is present, do not use the vaccine. Dispose of it safely.
If the vaccine passes the visual inspection and contains the correct dosage, administer intramuscularly.

Post-Administration Best Practices
Be sure to manage reactions immediately after administering the vaccines and store remaining doses carefully. Following these post-vaccine administration guidelines will ensure your practice is ready and able to follow up with each patient’s next dose if it is required for the vaccine manufacturer you are using.
Managing Adverse Reactions
Even if a patient has no history of allergies or severe allergic reactions, be sure to have all the appropriate treatments ready and accessible in the event of an acute anaphylactic reaction after the administration of a COVID-19 vaccine. Observe all patients for 15 minutes after administering a vaccine. Observe higher risk patients for 30 minutes.
If the patient has no immediate reaction, they may leave the practice, however they may still experience adverse reactions following vaccination. Inform your patients about these reactions and what they should do if they become serious.
Pfizer-BioNTech vaccine reactions may include:
- Pain, redness and/or swelling at the injection site
- Fatigue
- Headache
- Muscle pain
- Chills
- Joint pain
- Fever
- Nausea
- Swollen glands
Moderna vaccine reactions may include:
- Pain, redness and/or swelling at the injection site
- Fatigue
- Headache
- Myalgia
- Arthralgia
- Chills
- Nausea and/or vomiting
- Fever
Johnson & Johnson vaccine reactions may include:
- Pain, redness and/or swelling at the injection site
- Fatigue
- Headache
- Muscle pain
- Chills
- Fever
- Nausea
Scheduling the Second Dosage
Administering the second dose of the COVID-19 vaccine is essential to the efficacy of the vaccination program if you are using the Pfizer-BioNTech or Moderna vaccines. The Johnson & Johnson vaccine requires only one dose.
- The Pfizer-BioNTech vaccine requires a second 0.3 milliliter dose after 21 days
- The Moderna vaccine requires a second 0.5 milliliter dose after 28 days
The second dose for either of these vaccines should not take place earlier than recommended.
All COVID-19 vaccines should also be administered alone. Any other required vaccines should be administered at least 14 days before or after the administration of a COVID-19 vaccine, unless the other vaccines provide benefits that outweigh the risks of co-administration. This includes:
- Vaccines containing tetanus toxoid used for wound management
- Rabies vaccines for post-vaccination prophylaxis
- Vaccines required for admission or onboarding to a long-term care facility
- Vaccines administered for disease outbreaks
Preventing Inventory Waste
After administering COVID-19 vaccines, it’s essential to store and track the remaining doses carefully. Incorrect storage can lead to ineffective vaccines, wastage of limited supplies, and interruptions to vaccine scheduling. This can set back the immunization program and put your patients at risk. Recording doses and handling each vaccine correctly are key.
Pfizer-BioNTech
After reconstituting the Pfizer-BioNTech vaccine, record the time and date on the vaccine label. If left at room temperature, all doses in the vial must be administered within 6 hours to avoid bacterial contamination. After 6 hours, the vials must be discarded.
Since you cannot return the vaccine to ultra-cold temperature storage, you need to plan out all of your vaccination appointments carefully. One multi-dose vial contains 6 doses depending on the needle and syringe. Have a few patients on standy-by in the event that not everyone attends their appointment. If you have any doses left and no stand-bys, try to offer these doses to priority groups, but above all else, do not waste the vaccine.
Moderna
After administering the first dose from a Moderna vial, record the date and time on the vaccine label. If left at room temperature, all doses in a Moderna vial must be administered within 12 hours and cannot be refrozen.
Any vials that fall outside the temperature range or have not been used within 12 hours of puncture at room temperature must be discarded. One multi-dose vial contains 10 doses. To avoid wasting the vaccine, only thaw the vials that you need and have patients on stand-by to receive the vaccine in case there are any no-shows for appointments.
Johnson & Johnson
After puncturing the Johnson & Johnson vial to prepare the vaccine, record the date and time on the vaccine label. Unpunctured vials can be left at room temperature for up to 12 hours, while punctured vials can be left at room temperature for up to 6 hours. You cannot freeze this vaccine once punctured.
Discard any vaccines that fall out of these temperature ranges or have not been used within these timeframes. Each multi-dose vial contains 5 doses and only one dose is required for vaccination, so do not waste doses.

How to Simplify COVID-19 Vaccine Administration
Efficient and effective vaccine storage has never been more critical to your practice. You need a reliable storage system to keep different vaccines safe at different temperatures, and you also need a way to track these vaccines to manage availability and prevent waste. A centralized system can streamline the entire process so you can focus on ensuring the success of your COVID-19 immunization program.
The AccuVax Vaccine Management System has been purpose-built for vaccine storage and workflows. This technology guarantees temperature control for frozen and refrigerated vaccines within the same unit and includes a built-in battery pack to maintain temperatures for up to 15 hours after a power outage. Thanks to the real-time inventory tracking, you can see how many vaccines you have available. All the vials are automatically rotated by expiry so you can ensure COVID-19 vaccine administration guidelines quickly and easily.
The AccuShelf Inventory Management System can also simplify workflows while maintaining cold storage requirements. A wireless scanner allows you to record the lot, expiry, and dose of every vaccine, while the integrated digital temperature loggers provide real-time temperature monitoring. With complete vaccination data and automatic updates, you can keep track of your inventory, provide your patients with vaccines at the right time, and avoid waste.
To see how the AccuVax and AccuShelf systems can ensure the correct COVID-19 vaccine administration guidelines, request a demo now.